Aspergillomarasmine, a promising adjuvant to overcome antibiotic resistance
DOI:
https://doi.org/10.69998/e5vh9551Keywords:
Aspergillomarasmine A, Antibiotic resistance, Metallo-beta-lactamase inhibitor, Beta-lactam antibioticsAbstract
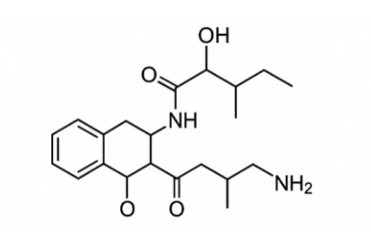
Bacterial resistance to antibiotics poses a significant public health challenge. One mechanism by which bacteria acquire resistance is the synthesis of beta-lactamases that inhibit the activity of beta-lactam antibiotics. Metallo-beta-lactamases (MBL) are a class of these enzymes for which there are currently no clinically available inhibitors to restore the efficacy of beta-lactam antibiotics. Aspergillomarasmine A (AMA), a fungal metabolite, has emerged as a compelling candidate, selectively chelating Zn²⁺ to inactivate MBLs and restore β-lactam activity. This review integrates current knowledge gathered from electronic databases, including ScienceDirect, PubMed, Scopus, Web of Science, and Google Scholar, on the mechanism of action and potential role of AMA in antibiotic/adjuvant co-therapy. Evidence to date suggests that AMA’s unique mode of action may limit the development of rapid resistance, although its clinical efficacy remains unproven. Accelerating AMA’s preclinical and clinical evaluation is imperative to translate its promise role into a novel strategy against multidrug-resistant bacteria.
Downloads
References
Boyd SE, Livermore DM, Hooper DC, Hope WW. 2020. Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother 64 (10): e00397-20. https://doi.org/10.1128/AAC.00397-20
Brem J., van Berkel S. S., Zollman D., Lee S. Y., Gileadi O., McHugh P. J., Walsh T. R., McDonough M. A., Schofield C. J. 2016. Structural basis of metallo—lactamase inhibition by captopril stereoisomers. Antimicrob Agents Chemother. 60:142 150. doi:10.1128/AAC.01335-15
European Centre for Disease Prevention and Control. Carbapenem-resistant Enterobacterales, third update. 2025. ECDC: Stockholm; 2025. DOI 10.2900/8752612
Hall B. G., Barlow M. 2005. Revised Ambler classification of β-lactamases, Journal of Antimicrobial Chemotherapy. 55 (6), 1050–1051, https://doi.org/10.1093/jac/dki130
He Y., Zhou S., Sun W., Li Q., Wang J., Zhang J. 2022. Emerione A, a novel fungal metabolite as an inhibitor of New Delhi metallo-β-lactamase 1, restores carbapenem susceptibility in carbapenem-resistant isolates. Journal of Global Antimicrobial Resistance: 216–222. https://doi.org/10.1016/j.jgar.2021.12.019
Ju L. C., Cheng Z., Fast W., Bonomo R. A., Crowder M. W. 2018. The Continuing Challenge of Metallo-β-Lactamase Inhibition: Mechanism Matters. Trends Pharmacol Sci. 39(7), 635–647. doi:10.1016/j.tips.2018.03.007
Kang S. J., Kim D. H., Bong-Jin Lee B J. 2024. Metallo-β-lactamase inhibitors: A continuing challenge for combating antibiotic resistance. Biophysical Chemistry 309: 107228. doi.org/10.1016/j.bpc.2024.107228
King A. M., Reid-Yu S. A., Wang W., King D. T., De Pascale G., Strynadka N.C., Walsh T. R., Coombes B. K., Wright G. D. 2014. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 510, 503 – 519. https://doi:10.1038/nature13445
Koteva K., Sychantha D., Rotondo C. M., Hobson C., Britten J. F., Wright G. D. 2022. Three-Dimensional Structure and Optimization of the Metallo-β-Lactamase Inhibitor Aspergillomarasmine A. ACS Omega. 7, 4170−4184. http://pubs.acs.org/journal/acsodf
Li X., Zhao J., Zhang B., Duan X., Jiao J., Wu W., Zhou Y., Wang H. 2022. Drug development concerning metallo-β-lactamases in gram-negative bacteria. Front. Microbiol. 13, 959107. doi: 10.3389/fmicb.2022.959107
Longhi, C., Maurizi, L., Conte, A. L., Marazzato, M., Comanducci, A., Nicoletti, M., Zagaglia, C. 2022. Extraintestinal Pathogenic Escherichia coli: Beta-Lactam Antibiotic and Heavy Metal Resistance. Antibiotics, 11(3), https://doi.org/10.3390/antibiotics11030328
Liu M., Wu C.,1, Zhang X., Wang Y., Wei Y., Hu Y., Sun Z., Chen C., Ma Y. 2025. Discovery of a dual-action compound for metallo-β-lactamase inhibition and biofilm clearance to reverse CRE resistance. Journal of Inorganic Biochemistry 273: 113028. https://doi.org/10.1016/j.jinorgbio.2025.113028
Mancuso G., Midiri A., Gerace E., Biondo C. 2021. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens. 10, 1310. https://doi.org/10.3390/ pathogens10101310
Mojica M. F., Rossi M-A., Vila A. J., Bonomo R. A. 2022. The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect Dis. 22, e28–34. https://doi.org/10.1016/S1473-3099(20)30868-9
Palacios A. R., Rossi M-A, Mahler G. S., Vila A. J. 2020. Metallo--Lactamase Inhibitors Inspired on Snapshots from the Catalytic Mechanism. Biomolecules. 10, 854. doi:10.3390/biom10060854
Rotondo C. M., Sychantha D., Koteva K., Wright G. D. 2020. Suppression of beta-lactam resistance by aspergillomarasmine A is influenced by both the metallo-beta-lactamase target and the antibiotic partner. Antimicrob. Agents Chemother. 64, e01386-19. doi: 10.1128/AAC.01386-19
Rotondo C. M., Wright G D. 2024. Efficacy of aspergillomarasmine A/meropenem combinations with and without avibactam against bacterial strains producing multiple β-lactamases. Antimicrobial Agents and Chemotherapy 68 ( 9). DOI 10.1128/aac.00272-24
Sychantha D., Rotondo C. M., Tehrani K. H. M. E., Martin N. I., Wright G. D. 2021. Aspergillomarasmine A inhibits metallo-β-lactamases by selectively sequestering Zn2+. J. Biol. Chem. 297(2), 100918. https://doi.org/10.1016/j.jbc.2021.100918.
WHO. 2023. Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/ antimicrobial-resistance (Accessed 21/01/2025)
Yoshizumi A., Ishii Y., Kimura S., Saga T., Harada S., Yamaguchi K., Tateda K., Livermore DM, Woodford N, Livermore DM. 2013. Efficacies of calcium–EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J Infect Chemother 19: 992–995. https://doi.org/10.1007/s10156-012-0528-y
Zahir I., Houari A., Bahafid W., Iraqui M., Ibnsouda S. 2013. A novel Alcaligenes faecalis antibacterial-producing strain isolated from a Moroccan tannery waste. African Journal of Microbiology Research. 7(47), 5314-5323. DOI: 10.5897/AJMR2013.6029
Zhang Y., Yang S., Deng Z., Song H., Xie N., Tian Y., Qin S., Liu J., Guo Y., Wang D., Liu J., Wu C., Shen J.,Ma S., Wang Y., Liu D. 2025. Antifungal agent tavaborole as a potential broad-spectrum serine and metallo-β-lactamases inhibitor. eBioMedicine;116: 105754. https://doi.org/10.1016/j.ebiom.2025.105754
Downloads
Published
Data Availability Statement
The datasets presented in this study are available upon reasonable request from the corresponding author.
Issue
Section
License
Copyright (c) 2025 Ilham Zahir (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




